2.1 Definition
2.2 Initiating a SOP
2.3 Preparation of SOPs
2.4 Administration, Distribution, Implementation
2.5 Laboratory notebook
2.6 Relativization as encouragement
SOPs
- User Manuals And Sops Contain Exactly The Same Components Pdf
- User Manuals And Sops Contain Exactly The Same Components Of Life
- User Manuals And Sops Contain Exactly The Same Components Of Water
Definition of an SOP — (Standard Operating Procedures) SOPs are written steps to explain good manufacturing practices (GMP), plant safety routines, financial controls to secure assets, or IT security measures that employees are to follow. SOPs are step by step procedures on how to do something that is critical to quality, critical to safe operations, or critical to security. Centrifuge Standard Operating Procedures. Exactly the same amount. If you only have one sample, you must use a second tube/bottle containing water and ensure that the weight of the two vessels is the same. The last 2 pages are reference pages for the user to keep. Components of an SOP. Terms in this set (10) Purpose. Why this SOP exists. To what or whom the SOP applies to. What will be used in the SOP. Warnings about anything harmful in the SOP.
2.1 Definition
An important aspect of a quality system is to work according to unambiguous Standard Operating Procedures (SOPs). In fact the whole process from sampling to the filing of the analytical result should be described by a continuous series of SOPs. A SOP for a laboratory can be defined as follows:
Knowing the difference between procedures and work instructions can help you understand the documentation process much better and, therefore, develop great procedure documentation. New Release of “How to Write a Policies and Procedures Manual” is now available.
'A Standard Operating Procedure is a document which describes the regularly recurring operations relevant to the quality of the investigation. The purpose of a SOP is to carry out the operations correctly and always in the same manner. A SOP should be available at the place where the work is done'.
A SOP is a compulsory instruction. If deviations from this instruction are allowed, the conditions for these should be documented including who can give permission for this and what exactly the complete procedure will be. The original should rest at a secure place while working copies should be authenticated with stamps and/or signatures of authorized persons.
Several categories and types of SOPs can be distinguished. The name 'SOP' may not always be appropriate, e.g., the description of situations or other matters may better designated protocols, instructions or simply registration forms. Also worksheets belonging to an analytical procedure have to be standardized (to avoid jotting down readings and calculations on odd pieces of paper).
A number of important SOP types are:
- Fundamental SOPs. These give instructions how to make SOPs of the other categories.
- Methodic SOPs. These describe a complete testing system or method of investigation.
- SOPs for safety precautions.
- Standard procedures for operating instruments, apparatus and other equipment.
- SOPs for analytical methods.
- SOPs for the preparation of reagents.
- SOPs for receiving and registration of samples.
- SOPs for Quality Assurance.
- SOPs for archiving and how to deal with complaints.
2.2 Initiating a SOP
As implied above, the initiative and further procedure for the preparation, implementation and management of the documents is a procedure in itself which should be described. These SOPs should at least mention:
a. who can or should make which type of SOP;
b. to whom proposals for a SOP should be submitted, and who adjudges the draft;
c. the procedure of approval;
d. who decides on the date of implementation, and who should be informed;
e. how revisions can be made or how a SOP can be withdrawn.
It should be established and recorded who is responsible for the proper distribution of the documents, the filing and administration (e.g. of the original and further copies). Finally, it should be indicated how frequently a valid SOP should be periodically evaluated (usually 2 years) and by whom. Only officially issued copies may be used, only then the use of the proper instruction is guaranteed.
In the laboratory the procedure for the preparation of a SOP should be as follows:
The Head of Laboratory (HoL) charges a staff member of the laboratory to draft a SOP (or the HoL does this himself or a staff member takes the initiative). In principle, the author is the person who will work with the SOP, but he or she should always keep in mind that the SOP needs to be understood by others. The author requests a new registration number from the SOP administrator or custodian (which in smaller institutes or laboratories will often be the HoL, see 2.4). The administrator verifies if the SOP already exists (or is drafted). If the SOP does not exist yet, the title and author are entered into the registration system. Once the writing of a SOP is undertaken, the management must actively support this effort and allow authors adequate preparation time.
In case of methodic or apparatus SOPs the author asks one or more qualified colleagues to try out the SOP. In case of execution procedures for investigations or protocols, the project leader or HoL could do the testing. In this phase the wording of the SOP is fine-tuned. When the test is passed, the SOP is submitted to the SOP administrator for acceptance. Revisions of SOPs follow the same procedure.
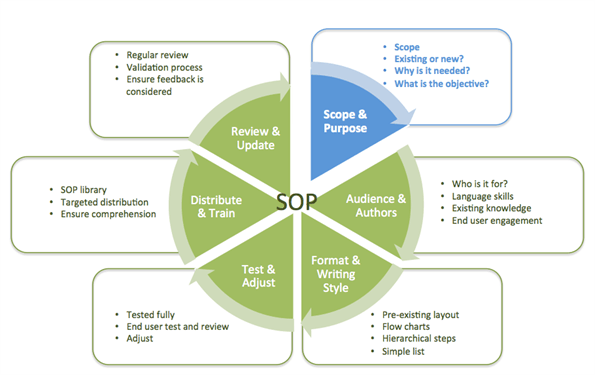
2.3 Preparation of SOPs
The make-up of the documents should meet a minimum number of requirements:
1. Each page should have a heading and/or footing mentioning:a. date of approval and/or version number;
b. a unique title (abbreviated if desired);
c. the number of the SOP (preferably with category);
d. page number and total number of pages of the SOP.
e. the heading (or only the logo) of originals should preferably be printed in another colour than black.
Categories can be denoted with a letter or combination of letters, e.g.:
- F for fundamental SOP- A or APP for apparatus SOP
- M or METH for analytical method SOP
- P or PROJ for procedure to carry out a special investigation (project)
- PROT for a protocol describing a sequence of actions or operations
- ORG for an organizational document
- PERS for describing personnel matters
- RF
 for registration form (e.g. chemicals, samples)
for registration form (e.g. chemicals, samples)- WS for worksheet (related to analytical procedures)
2. The first page, the title page, should mention:
a. general information mentioned under 2.3.1 above, including the complete title;b. a summary of the contents with purpose and field of application (if these are not evident from the title); if
desired the principle may be given, including a list of points that may need attention;
c. any related SOPs (of operations used in the present SOP);
d. possible safety instructions;
e. name and signature of author, including date of signing. (It is possible to record the authors centrally in a register);
f. name and signature of person who authorizes the introduction of the SOP (including date).
3. The necessary equipment, reagents (including grade) and other means should be detailed.
4. A clear, unambiguous imperative description is given in a language mastered by the user.
5. It is recommended to include criteria for the control of the described system during operation.
6. It is recommended to include a list of contents particularly if the SOP is lengthy.
7. It is recommended to include a list of references.
2.4 Administration, Distribution, Implementation
From this description it would seem that the preparation and administration of a SOP and other quality assurance documentation is an onerous job. However, once the draft is made, with the use of word processors and a simple distribution scheme of persons and departments involved, the task can be considerably eased.
A model for a simple preparation and distribution scheme is given in Figure 2-1. This is a relation matrix which can not only be used for the laboratory but for any department or a whole institute. In this matrix (which can be given the status of a SOP) can be indicated all persons or departments that are involved with the subject as well as the kind of their involvement. This can be indicated in the scheme with an involvement code. Some of the most usual involvements are (the number can be used as the code):
1. Taking initiative for drafting
2. Drafting the document
3. Verifying
4. Authorizing
5. Implementing/using
6. Copy for information
7. Checking implementation
8. Archiving
Fig. 2-1. Matrix of information organization (see text).
There is a multitude of valid approaches for distribution of SOPs but there must always be a mechanism for informing potential users that a new SOP has been written or that an existing SOP has been revised or withdrawn.
It is worthwhile to set up a good filing system for all documents right at the outset. This will spare much inconvenience, confusion and embarrassment, not only in internal use but also with respect to the institute's management, authorities, clients and, if applicable, inspectors of the accreditation body.
The administrator responsible for distribution and archiving SOPs may differ per institute. In large institutes or institutes with an accredited laboratory this will be the Quality Assurance Officer, otherwise this may be an officer of the department of Personnel & Organization or still someone else. In non-accredited laboratories the administration can most conveniently be done by the head of laboratory or his deputy. The administration may be done in a logbook, by means of a card system or, more conveniently, with a computerized database such as PerfectView or Cardbox. Suspending files are very useful for keeping originals, copies and other information of documents. The most logic system seems to make an appropriate grouping into categories and a master index for easy retrieval. It is most convenient to keep these files at a central place such as the office of the head of laboratory. Naturally, this does not apply to working documents that obviously are used at the work place in the laboratory, e.g., instrument logbooks, operation instruction manuals and laboratory notebooks.
The data which should be stored per document are:
- SOP number
- version number
- date of issue
- date of expiry
- title
- author
- status (title submitted; being drafted; draft ready; issued)
- department of holders/users
- names of holders
- number of copies per holder if this is more than one
- registration number of SOPs to which reference is made
- historical data (dates of previous issues)
The SOP administrator keeps at least two copies of each SOP; one for the historical and one for the back-up file. This also applies to revised versions. Superseded versions should be collected and destroyed (except the copy for the historical file) to avoid confusion and unauthorized use.
Examples of various categories of SOPs will be given in the ensuing chapters. The contents of a SOP for the administration and management of SOPs can be distilled from the above. An example of the basic format is given as Model F 002.
2.5 Laboratory notebook
Unless recorded automatically, raw data and readings of measurements are most conveniently written down on worksheets that can be prepared for each analytical method or procedure, including calibration of equipment. In addition, each laboratory staff member should have a personal Notebook in which all observations, remarks, calculations and other actions connected with the work are recorded in ink, not with a pencil, so that they will not be erased or lost. To ensure integrity such a notebook must meet a few minimum requirements: on the cover it must carry a unique serial number, the owner's name, and the date of issue. The copy is issued by the QA officer or head of laboratory who keeps a record of this (e.g. in his/her own Notebook). The user signs for receipt, the QA officer or HoL for issue. The Notebook should be bound and the pages numbered before issue (loose-leaf bindings are not GLP!). The first one or two pages can be used for an index of contents (to be filled in as the book is used). Such Notebooks can made from ordinary notebooks on sale (before issue, the page numbering should then be done by hand or with a special stamp) or with the help of a word processor and then printed and bound in a graphical workshop.
The instructions for the proper use of a laboratory notebook should be set down in a protocol, an example is given as Model PROT 005. A model for the pages in a laboratory notebook is given.
2.6 Relativization as encouragement
In the Preface it was stated that documentation should not be overdone and that for the implementation of all new Quality Management rules the philosophy of a step-by-step approach should be adopted. It is emphasized that protocols and SOPs, as well as the administration involved, should be kept as simple as possible, particularly in the beginning. The Quality Management system must grow by trial and error, with increasing experience, by group discussions and with changing perceptions. In the beginning, attention will be focused on basic operational SOPs, later shifting to record keeping (as more and more SOPs are issued) and filling gaps as practice reveals missing links in the chain of Quality Assurance. Inevitably problems will turn up. One way to solve them is to talk with people in other laboratories who have faced similar problems.
Do not forget that Quality Management is a tool rather than a goal. The goal is quality performance of the laboratory.
SOPs
F 002 - Administration of Standard Operating Procedures
PROT 005 - The Use of Laboratory Notebooks
Model page of Laboratory Notebook
F 002 - Administration of Standard Operating Procedures
LOGO | STANDARD OPERATING PROCEDURE | Page: 1 # 2 | |
Model: F 002 | Version: 1 | Date: 95-06-21 | |
Title: Administration of Standard Operating Procedures | File: | ||
1. PURPOSE
To give unambiguous instruction for proper management and administration of Standard Operating Procedures as they are used in the Regional Soil Survey Institute (RSSI).
2. PRINCIPLE
Standard Operating Procedures are an essential part of a quality system. For all jobs and duties relevant operating procedures should be available at the work station. To guarantee that the correct version of the instruction is used copying Standard Operating Procedures is prohibited. Standard Operating Procedures are issued on paper with the heading printed in green.
3. FIELD OF APPLICATION
Generally for use in the quality system of RSSI but more specifically this instruction is for use in the Chemistry Department.
4. RELATED SOPs
- F 011 Hp laserjet pro m130nw user manual. Manuals or user guides for your HP LaserJet Pro MFP M130nw. | The preparation of SOPs for apparatus View and Download HP Pavilion dv7 maintenance and service manual online. HP Pavilion dv7 Notebook PC. Pavilion dv7 Laptop pdf manual download. View and Download HP ENVY dv7-7200 maintenance and service manual online. HP ENVY dv7 Notebook PC Maintenance and Service Guide IMPORTANT! This document is intended for HP authorized service providers only. ENVY dv7-7200 Laptop pdf manual download. Also for: Envy dv7-7300, Envy dv7. Hp envy dv7 notebook pc user manual. View and Download HP ENVY dv7-7200 user manual online. User Guide - Windows 8. ENVY dv7-7200 Laptop pdf manual download. Also for: Envy dv7-7300, Envy dv6-7200. 1 Product description Category Description Computer models equipped with an AMD processor Computer models equipped with an Intel processor Product Name HP ENVY dv7 Notebook PC √√ Processors AMD® A10-4600M 3.20-GHz processor (1600-MHz FSB, 4.0-MB L2 cache. HP ENVY dv7-7200 Maintenance And Service Manual (129 pages). Hp envy dv7 notebook pc maintenance and service guide important! This document is intended for hp. |
- F 012 | The preparation of SOPs for methods |
- PROJ 001 | The preparation of SOPs for special investigations |
User Manuals And Sops Contain Exactly The Same Components Pdf
5. REQUIREMENTS
Database computer program, PerfectView or Cardbox
6. PROCEDURE
6.1 Administration
The administration of SOPs for the Chemistry Department can be done by the Head of Laboratory.
6.2 Initiating new SOP
(See these Guidelines, 2.2)
6.3 Revision of SOPs
(see these Guidelines, 2.2)
Author: | Sign.: |
QA Officer (sign.): | Date of Expiry: |
6.5 Distribution of SOPs
When the Sop fulfils all the necessary requirements it is printed. The author hands over the manuscript (or the floppy disk with text) to the SOP administrator who is responsible for the printing. The number of copies is decided by him/her and the author. Make matrix of distribution (see Guidelines for Quality Management Fig. 2-1).
The author (or his successor) signs all copies in the presence of the administrator before distribution. As the new copies are distributed the old ones (if there was one) are taken in. For each SOP a list of holders is made. The holder signs for receipt of a copy. The list is kept with the spare copies.
Copying SOPs is forbidden. Extra copies can be obtained from the SOP administrator.
Users are responsible for proper keeping of the SOPs. If necessary, copies can be protected by a cover or foil, and/or be kept in a loose-leaf binding.
7. ARCHIVING
Proper archiving is essential for good administration of SOPs. All operating instructions should be kept up-to-date and be accesible to personnel. Good Laboratory Practice requires that all documentation pertaining to a test or investigation should be kept for a certain period. SOPs belong to this documentation.
8. REFERENCES
Mention here the used Standards and other references for this SOP.
PROT 005 - The Use of Laboratory Notebooks
LOGO | STANDARD OPERATING PROCEDURE | Page: 1 # 2 | |
Model: F 002 | Version: 1 | Date: 95-11-28 | |
Title: The Use of Laboratory Notebooks | File: | ||
1. PURPOSE
To give instruction for proper lay-out, use and administration of Laboratory Notebooks in order to guarantee the integrity and retrievability of raw data (if no preprinted Work Sheets are used), calculations and notes pertaining to the laboratory work.
2. PRINCIPLE
Laboratory Notebooks may either be issued to persons for personal use or to Study Projects for common use by participating persons. They are used to write down observations, remarks, calculations and other actions in connection with the work. They may be used for raw data but bound preprinted Work Sheets are preferred for this.
3. RELATED SOPs
Administration of SOPs | |
PROJ 001 | The preparation of SOPs for Special Investigations |
4. REQUIREMENTS

Bound notebooks with about 100-150 consecutively numbered pages. Any binding which cannot be opened is suitable; a spiral binding is very convenient.
Both ruled and squared paper can be used. On each page provisions for dating and signing for entries, and signing for verification or inspection may be made.
5. PROCEDURE
5.1 Issue
Notebooks are issued by or on behalf of the Head of Laboratory who keeps a record of the books in circulation (this record may have a format similar to a Laboratory Notebook or be part of the HoL's own Notebook).
On the cover, the book is marked with an assigned (if not preprinted) serial number and the name of the user (or of the project). On the inside of the cover the HoL writes the date of issue and signs for issue. The user (or Project Leader) signs the circulation record for receipt.
5.2 Use
All entries are dated and made in ink. The person who makes the entry signs per entry (in project notebooks) or at least per page (in personal notebooks). The Head of Laboratory (and/or Project Leader) may inspect or verify entries and pages and may sign for this on the page(s) concerned.
If entries are corrected, this should be lined out with a single line so that it is possible to see what has been corrected. Essential corrections should be initialed and dated and the reason for correction stated. Pages may not be removed; if necessary, a whole page may be deleted by a diagonal line.
Author: | Sign.: |
QA Officer (sign.): | Date of Expiry: |
5.3 Withdrawal
When fall, the Notebook is exchanged for a new one. The HoL is responsible for proper archiving. A notebook belonging to a Study Project is withdrawn when the study is completed.
When an employee leaves the laboratory for another post (s)he should hand in her/his notebook to the HoL
6. ARCHIVING
The Head of Laboratory is custodian of the withdrawn Laboratory Notebooks. They must remain accessible for inspection and audit trailing,
View and Download Sharp SPC900 user instructions online. RADIO CONTROLLED WIRELESS THERMO CLOCK. SPC900 Weather Station pdf manual download. My SHARP Atomic Clock Model SPC 900 the background has turned Black, couldn’t see the time display. How to set it right. Sharp Radio Controlled Atomic Thermo Clock Instruction Manual. SPC900 Clock pdf manual download. Sharp SPC900 Instruction Manual. Sharp radio controlled atomic thermo clock instruction manual. Also See for SPC900. User instructions - 11 pages. Most useful pages: More. Time zone Settings Time. Sharp atomic clock model spc900 user manual.
7. REFERENCES
Model page of Laboratory Notebook
Date/Signature | SUBJECT |
Digiprog 3 V4.94 most popular universal mileage correction tool, work on almost cars in the markets. Digiprog iii support multi-language and update via serial number directly. Kindly notice: do not connect dp3 to computer, otherwise you will get blue screen problem. This post answers all the detailed questions about DigiProg 3 Milleage programmer, you can free download Technical Documentation center of DigiProg 3 Milleage programmer, including: user manual, update guide, specification, inside structure, solution for Blue / White screen error, good or bad tested vehicle results and supported Vehicles list etc. Jul 17, 2015 Digiprog 3 III odometer correction 2014 manual free download. Still Digiprog 3 odometer correction tool new beginners are seeking user manual instructions. Obd365 here provides both old and new instruction with English and German language. V4.94 digiprog 3. Digiprog 3 v4 94 user manual online. Aug 15, 2018 3) Step-by-step procedure on how to correct mileage on each car makes. 4) DIGIPROG 3 cable pinouts text file. Pictures mean a lot. The Digiprog user manual comes with default German language and English language. 2014 New digiprog 3 DP3 odometer master user manual on mediafire. May 30, 2016 Free download Digiprog 3 odometer correction 2014 manual - 19,438 views Digiprog 3 update instruction and vehicle list free download - 16,454 views Using Digiprog 3 V4.94 to program EEPROM Guide - 14,312 views. | |
Verified by:
Signature: ______________________ | WO/Test no. __________________ |
Date: __________________________ | File: _________________________ |
A user guide, also commonly called a technical communication document or manual, is intended to give assistance to people using a particular system.[1] It is usually written by a technical writer, although user guides are written by programmers, product or project managers, or other technical staff, particularly in smaller companies.[2]
User guides are most commonly associated with electronic goods, computer hardware and software, although they can be written for any product.[3]
Most user guides contain both a written guide and associated images. In the case of computer applications, it is usual to include screenshots of the human-machine interface(s), and hardware manuals often include clear, simplified diagrams. The language used is matched to the intended audience, with jargon kept to a minimum or explained thoroughly.
Contents of a user manual[edit]
The sections of a user manual often include:
- A cover page
- A title page and copyright page
- A preface, containing details of related documents and information on how to navigate the user guide
- A contents page
- A Purpose section. This should be an overview rather than detail the objective of the document
- An Audience section to explicitly state who is not as well as who is required to read, including optionals
- A Scope section is crucial as it also serves as a disclaimer, stating what is out-of-scope as well as what is covered
- A guide on how to use at least the main function of the system
- A troubleshooting section detailing possible errors or problems that may occur, along with how to fix them
- A FAQ (Frequently Asked Questions)
- Where to find further help, and contact details
- A glossary and, for larger documents, an index
History[edit]
User guides have been found with ancient devices. One example is the Antikythera Mechanism[4], a 2,000 year old Greek analogue computer that was found off the coast of the Greek island Antikythera in the year 1900. On the cover of this device are passages of text which describe the features and operation of the mechanism.
As the software industry was developing, the question of how to best document software programs was undecided. This was a unique problem for software developers, since users often became frustrated with current help documents[5]. Some considerations for writing a user guide that developed at this time include:
- the use of plain language[5]
User Manuals And Sops Contain Exactly The Same Components Of Life
- length and reading difficulty[5]
- the role of printed user guides for digital programs[6]
- user-centered design[6]
Computer software manuals and guides[edit]
User manuals and user guides for most non-trivial software applications are book-like documents with contents similar to the above list. They may be distributed either in print or electronically. Some documents have a more fluid structure with many internal links. The Google Earth User Guide[7] is an example of this format. The term guide is often applied to a document that addresses a specific aspect of a software product. Some usages are Installation Guide, Getting Started Guide, and various How to guides. An example is the Picasa Getting Started Guide.[8]
In some business software applications, where groups of users have access to only a sub-set of the application's full functionality, a user guide may be prepared for each group. An example of this approach is the Autodesk Topobase 2010 Help[9] document, which contains separate Administrator Guides, User Guides, and a Developer's Guide.
User Manuals And Sops Contain Exactly The Same Components Of Water
References[edit]
- ^'Online Technical Writing: User Guides'. hcexres@io.com. Retrieved 13 August 2009.
- ^Gary Blake and Robert W. Bly, The Elements of Technical Writing, pg. 143. New York: Macmillan Publishers, 1993. ISBN0020130856
- ^'Manuals Brain - all useful manuals at one place!'. manualsbrain.com. Retrieved 2017-08-15.
- ^'Boffins decipher manual for 2,000-year-old Ancient Greek computer'. Retrieved 2018-11-29.
- ^ abcChafin, Roy (January 1982). 'User Manuals: What Does the User Really Need?'. SIGDOC '82 Proceedings of the 1st annual international conference on systems documentation: 36–39 – via ACM Digital Library.
- ^ abMcKee, John (August 1986). 'Computer User Manuals in Print: Do They Have a Future?'. ACM SIGDOC Asterisk Journal of Computer Documentation. 12: 11–16 – via ACM Digital Library.
- ^'Google Earth User Guide'. Google. 4 June 2009. Retrieved 13 August 2009.
- ^'Getting Started with Picasa: Getting Started Guide'. Google. 15 June 2009. Retrieved 13 August 2009.
- ^'Autodesk Topobase 2010 Help'. Autodesk. Retrieved 13 August 2009.
- ^Manualdevices - Free User Manual 'Manualdevices - Free User Manual ', Retrieved on 01 August 2019.